Tp chemistry II 18-24-28 (2023-2024)
Topic outline
-
-
Forum
-
Forum
-
-
-
These practical work sessions are designed for 1st year Common Base students. The Chemistry II practical work module consists of four manipulations:
- Manipulation N°1 : Calorific Capacity & Specific Heat
- Manipulation N°2 : The Latent Heat of Fusion of the ice
- Manipulation N°3 : Determining enthalpies of reaction
- Manipulation N°4 : Chemical kinetics: AUTO-CATALYSIS
Each practical work session is accompanied by theoretical information, and questions are asked to check the acquisition and assimilation of knowledge. A report is submitted to the teacher within a maximum of 15 days from the date of the work session.
-
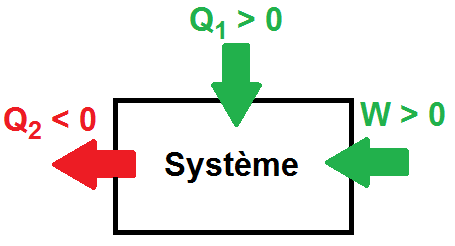
Thermodynamics is the science of energy exchange. It is used as much in physics as in chemistry and biology, relying in particular on mathematical tools. Its fundamental principles enable us to understand and predict energy variations between different interacting systems. Thermodynamics has many applications. These include thermal machines (car engines, refrigerators, heat pumps).
The calorific capacity C of a substance is the physical quantity that determines the amount of heat Q required to vary its temperature by a certain value ∆T. We know from experience that calorific capacity is an extensive property, in the sense that the calorific capacity of a given sample is directly proportional to its dimensions (it is faster to chauffer a glass of water than a barrel of water using the same heat source). The mass heat capacity is the calorific capacity related to the mass of the substance. It is an intensive quantity, because it depends only on the nature of the substance and not on its dimensions.
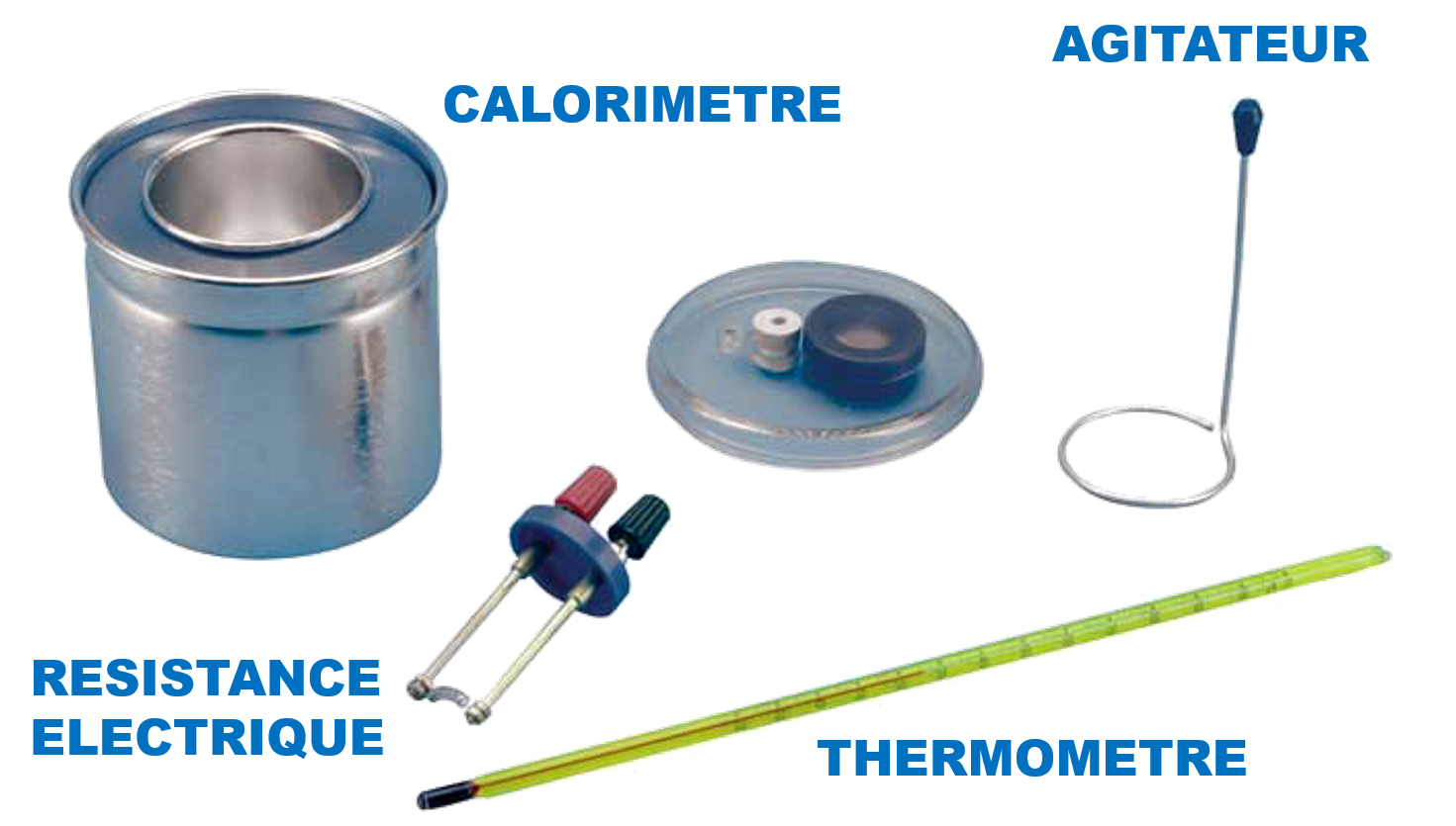
Objectives

1- Determine the calorific capacity of the calorimeter.
2- Determining the specific heat of a liquid (ethanol).
3- Determining the specific heat of a metal.
ENG
 FR
FR
-
Chat
-
Feedback
-
Forum
-
A given pure substance can exist in 4 states: solid, liquid, gas and plasma. The change of physical state requires an exchange of heat (energy) with the external environment.
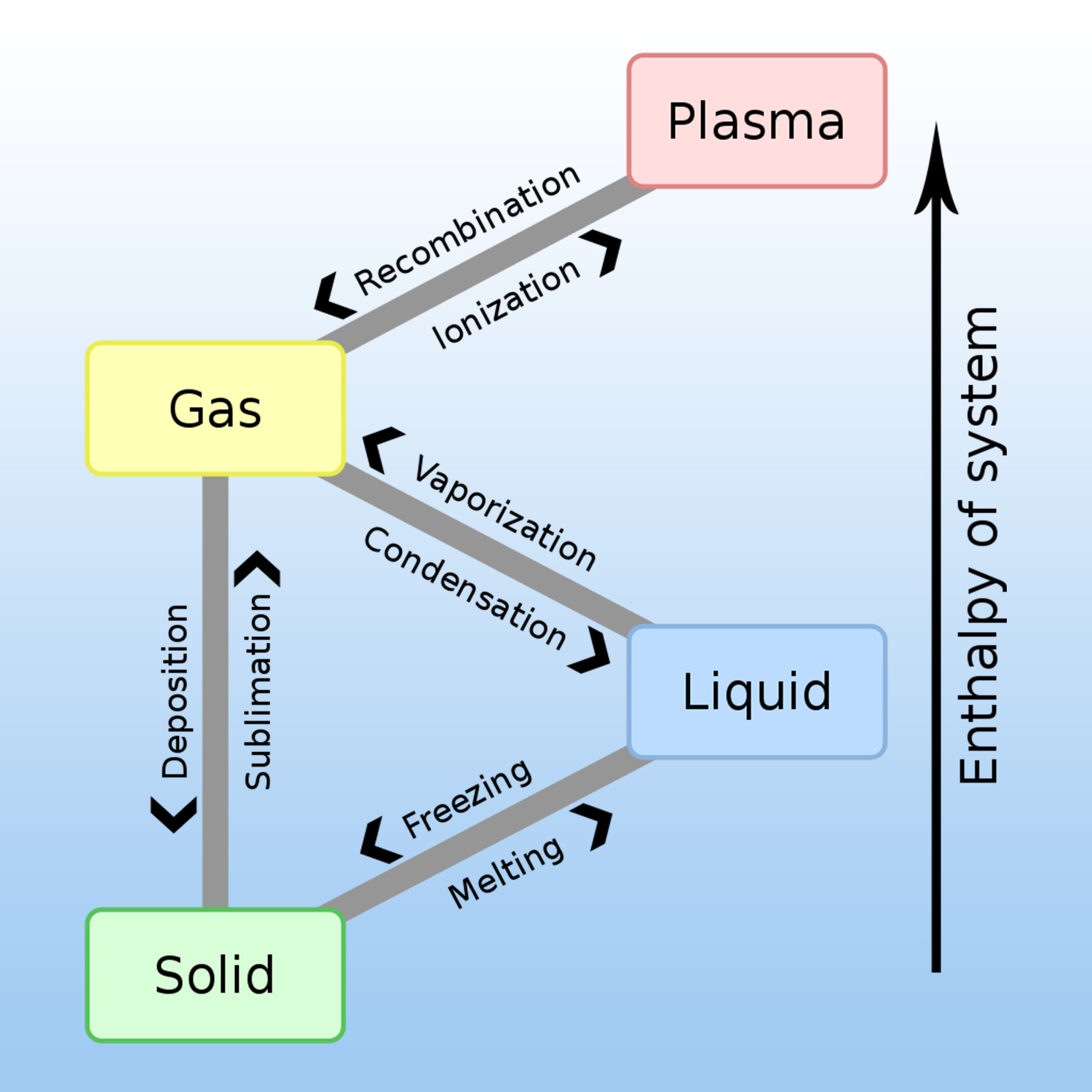
A physical change is produced when there is no transformation of matter, for example, liquid water that evaporates always remains water, H2O.
At constant pressure, once a pure substance (such as water) has reached its change-of-state temperature, it needs an additional quantity of energy to change state: this is the mass energy of change of state, also called the « latent heat of change of state », noted L. It is measured by the variation in thermal energy Q, such as :
Q = m . L m: the mass in Kg.
Objective

1- Study of change of state phenomenon.
2- Determination of the calorific capacity of the calorimeter (Ccal).
3- Determination of the latent heat of fusion of the ice (Lf).
ENG
 FR
FR
-
Chat
-
Feedback
-
Forum
-
