TP chemistry 1 2023-2024 ER Group 01
مخطط الموضوع
-
-
منتدى
الاختبار في الأسفل
-
-

- Teacher: HERIZI ABDALLAH
- days of work: From Tuesday toThursday from 8:00 a.m. - 5:00 p.m
- Course information :
Faculty : Technology
Department : COMMON BASE ST
students : 1st year Licence
Course Title : TP Chimie 02
Credit : 02
Coefficient :01
Duration of study : 10 weeks
Duratio of science : 02 hours
Block: B
Room : Lab: 5,7,8 and 9.
-
These practical work sessions are intended for students of the 1st year Common base, The practical work module of chemistry I are composed of five manipulations:
1st Manipulation: General notions (Safety, Measuring tools in the Laboratory)
2nd Manipulation: Preparation of solutions.
3rd Manipulation: Titration Colorimetric of a strong acid by a strong base.
4th Manipulation: Redox titration .
5th Manipulation: Acetic acid titration .
Each practical work is accompanied by theoretical information and questions are proposed, in order to control the acquisition and assimilation of knowledge. and each manipulation is sanctioned by a report given to the teacher within a maximum period of 15 days from the date of the manipulation.
-

So that the student will be able to provide regular work in this course, it is necessary to acquire certain prior knowledge, in particular like:
-
les chapitres
-
الصفحة
-
-
-
المحادثة
-
-
-
-
Working in a chemical laboratory requires the application of a certain number of safety rules; these rules are essential for the organization of work in a laboratory. When you enter the lab room for the first time, the student must know what to do, know how to dress and behave during a lab session, know the essential rules for handling equipment and chemicals, glassware commonly used and knowing how to write a report.
The chemical laboratory exposes students to risks due both to potentially toxic chemicals and to the materials used, which an experimenter must know to use them without danger. You must therefore be aware of the risks involved and do everything to protect others and yourself, while keeping in mind that the danger can come from others.
-
Some general safety rules.
- Some tools and products from the chemistry lab.
-
المحادثة
-
-
The precise of volumes measurement is a great importance in the laboratory. It can be carried out using a volumetric or graduated pipette, a graduated burette or a volumetric flask. We will show in this sequence how to prepare or dilute a solution using a volumetric flask to contain a precise volume of liquid.
-
Preparation of a solution by dissolving.
Preparation of a solution by dilution.
-
المحادثة
-
-
In everyday life, we regularly use acidic or basic solutions: descaler, vinegar, lemon juice, ammonia, soda, etc. An acid is strong if its reaction with water can be considered complete and only its conjugate base remains in solution. The reaction of this strong acid AH is then written as follows:
AH + H2O → A - + H3O+
The goal of any titration is to determine the unknown concentration (via amount of product) of one (titrated) species using another (titrant) species of known concentration. The equivalence point between the quantities of materials present and those introduced allows, after a few calculations, the determination of the concentration of the species sought. Several methods are possible to determine this equivalence point: the introduction of a colored indicator, the pH value, the conductance value of the solution.
-
Colorimetric determination of hydrochloric acid by sodium hydroxide.
Plot the graph representing the value of pH= f(VB).
-
المحادثة
-
-
The purpose of redox titration is to determining the normality of a reducing solution knowing that of the oxidizing solution. We propose to study the oxidation of the Fe2+ ion by the permanganate ion MnO4 - in an acid medium. This assay is called manganimetry. The oxidizing properties of the permanganate ion are at the origin of manganimetry. The oxidizing form MnO4 - is purple, the reducing form Mn2+ is colorless, allowing the equivalent point to be determined without using color indicators.
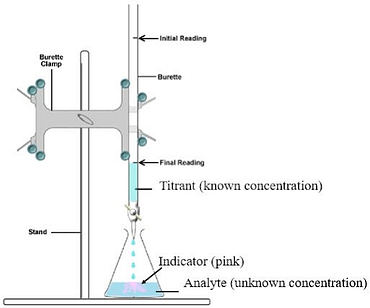
-

- Dosage du fer dans le sulfate ferreux par le permanganate de potassium.
- Ecrire les demi-réactions d’oxydo- réduction, préciser les couples redox.
- Determination of iron in ferrous sulphate by potassium permanganate.
- Write the oxidation-reduction half-reactions, specify the redox couples.
-
المحادثة
-
-
Acetic acid also called eisel (alcohol) made by acetic fermentation and used as a condiment or preservative. We will consider that vinegar is an aqueous solution of acetic acid with a density d close to 1. The purpose of this lab is to determine the degree of acidity D or (°) of a vinegar and defined as the mass of acid pure acetic or ethanoic contained in 100g of vinegar. For this, we will dose the acetic (ethanoic) acid contained in a known volume of vinegar using a strong base solution of known concentration: sodium hydroxide solution (NaOH).

During the assays, it is necessary to know precisely the concentration of the titrant solution (here NaOH). However, in most cases, the concentrations of the solutions are not strictly exact because:- the solution is made using a solid product whose purity is not guaranteed by the manufacturer.
- the solution is made using a product whose sampling (weighing, volume) cannot be precise.
- the chemical composition of the solution changes over time.
-

- How to control the quality of a product by dosage.
- Products tested different types of commercial vinegar.
-
المحادثة
-
الاختبارمفتوح: Friday، 15 December 2023، 8:00 AMمغلق: Friday، 15 December 2023، 6:00 PM
يرجى من الطلبة الافاضل الإجابة على أسئلة الاختبار الموجودة ادناه
* الاختبار متاح ابتداء من يوم الجمعة 15/ 12/ 2023.
* الاختبار يكون من الساعة 8:00 صباحا الى 18:00 مساء.
* مدة الاختبار 20 دقيقة.
* كل طالب لدية فرصة واحدة للإجابة لابد من اغتنامها.
* يرجى مراجعة الاعمال التطبيقية قبل البدأ في الاختبار.
* الأعمال التطبيقية المعنية بالإختبار هي من العملي من 7 و 11 و 15 و 19
* إضغط على استعراض الاختبار وأبدأ المحاولة



