Structure of matter / Chemistry (I)
Aperçu des sections
-

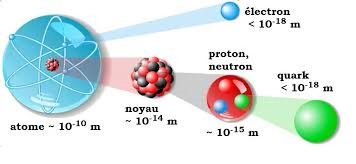
An atom consists of a central spherical nucleus and electrons moving in the vacuum around this nucleus. The nucleus is composed of particles called nucleons. These particles, comparable to microscopic beads, come in two types: protons and neutrons characterized by their mass and charge.
Electrons have a mass 1850 times smaller than nucleons, so it can be said that the mass of an electron is negligible compared to that of a neutron and a proton.
The charge of the electron is opposite to that of the proton. Since an atom is electrically neutral, it possesses an equal number of protons and electrons. The symbol for an atom is usually written as A X Z, where:
X represents the element symbol, which can be either a capital letter or a capital letter followed by a lowercase letter (e.g., H for hydrogen, He for helium).
A represents the number of nucleons (neutrons + protons).
Z represents the number of protons in the nucleus, known as the atomic number or proton number.
-
Forum
-

- Teacher : Dr Souheyla Chetioui
- Contact : souheyla.chetioui@univ-msila.dz
- Coefficient : 03 Credits: 06 Total Hour
- Volume : 67 hours and 30 minutes
- Required Weekly Study Hours : 4 hours and 30 minutes (3 hours of lectures and 1 hour 30 minutes of tutorials)
- Assessment Method : Diagnostic assessment + Formative assessment + Summative assessment.
-
Target audience

This course on "Matter Structure" (Chemistry 1) is intended for first-year university students in the fields of Science and Technology (ST) and Material Sciences (SM). It aligns with the curriculum of the first year of the common core for both domains. Its purpose is to provide a foundational set of knowledge on the laws and concepts necessary for understanding the structure of matter.
-
Matter is anything that possesses mass and occupies space.
All objects, air, water, oil... are matter; they are bodies.
These bodies can exist in different forms known as physical states: they can be solid, liquid, or gaseous depending on the degree of cohesion among the molecules that compose them.
-
The adopted plan proposes a gradual approach to general chemistry. This course is divided into eight chapters:
The first chapter of this course primarily covers the fundamental concepts of general chemistry, including a review of states of matter, atoms, molecules, and solutions.
Chapter II is dedicated to the classical conception of the atom and will discuss generalities about the structure of the atom and the various experiments that have revealed its constituents, namely, protons, neutrons, the nucleus, and electrons.
Chapter III deals with radioactivity and nuclear reactions.
Chapter IV focuses on the quantification of energy in the atomic model (wave-particle duality of light, hydrogen spectrum, and classical models of the atom) and the study of the wave model of the atom.
Chapter V is reserved for the periodic classification of elements, the evolution, and the periodicity of the physicochemical properties of elements.
Chapters VI, VII, and VIII are dedicated to chemical bonding: ionic bonding, covalent bonding, Lewis structure, the VSEPR method, and the hybridization of atomic orbitals.
-
Fichier
-
-
Fichier
-
Fichier
-
Atelier
-
Fichier
-
-
-
Fichier
-
-
Fichier
-
-
Fichier
-
Chat
-
Atelier
-
-
Fichier
-
Chat
-
Forum
-
-
Atelier
-
Chat
-
Forum
-
-
Fichier
-
Atelier
-
Forum
-
Test
-
References
1. R. Ouahès et B. Dévallez, Chimie générale, Editions OPU - Alger, 04 1993.
2. N. Glinka, Chimie générale, tome II, Editions MIR-Moscou, 1981.
3. G. Geiser, G. Delpin et P. Viaud, Chimie générale, Editions DELTA et SPES Lausanne, 1983.
4. F. Houma, Chimie générale, Editions LAMINE - Alger, 1995.
5. M. Fayard, Structure électronique atomes et molécules simples, Chimie physique, Edition HERMANN-Paris, Collection Méthodes, 1969.
6. R. et C. Ouahes, Chimie physique, Ellipses, 1995.
7. M. Gruia, M. Polisset, Chimie générale PCEM, Ellipses, 1993.
8. J.R. Kotz-Treichel, Chimie générale, Editions de boeck, 2006.
9. A. Durupthy, O. Durupthy, 1ière année Chimie PCSI, Hachette Supérieur, 2009.
10. P. Arnaud, Cours de Chimie générale, DUNOD, 2013.
11. http://www.chimie-briere.com.
-
