Structure de la maière-Exercices corrigés
مخطط الموضوع
-

Photo

Subject title:
Faculty: Technology
Department: common core
Cycle: 1st year Licence
Semester: 1
Credits: 3
Coefficients: 4
Volume: 02
Teacher:Mohamed Amine GHEBOULI
Email: mohamedamine.ghebouli@uni-msila.dz -
This standard, directed at first-year science and technology students in the first semester, aims to introduce
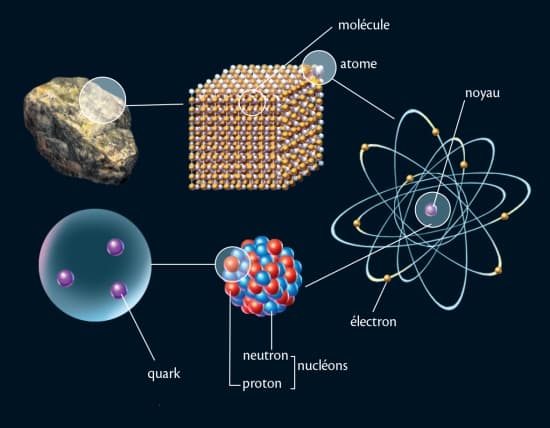
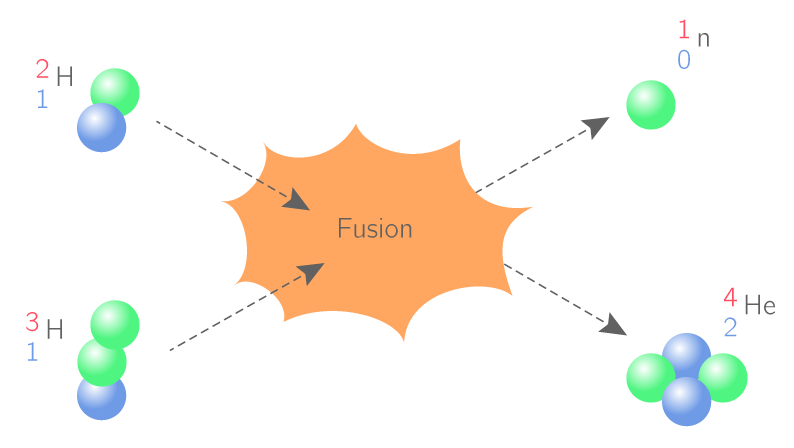
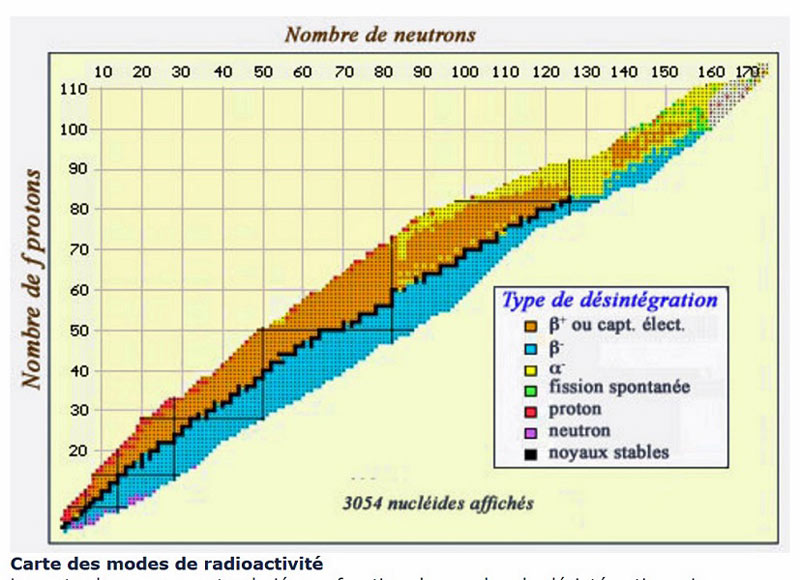
them to matter and its basic components, which are the atom. And the study of various physical and chemicalAt the end of this scale, the student will be able to... 1- Distinguish what matter is, its physical states, and its basic components 2- Different theories understand the experiments that allowed the discovery of the various components of matter 3- He applies various previous theories to radioactive nuclei and studies their effectiveness 4- Study of the Bohr atomic model
-
المحادثة
-
الإفادة
-
-

Question 1. A co-ordinate bond is formed by:
(a)sharing of electrons contributed by both the atoms
(b)complete transfer of electrons
(c)sharing of electrons contributed by one atom only (d) none of these
Question 2. The species CO, CN– and N2 are:
(a) isoelectronic (b) having coordinated bond
(c)having polar bond (d) having low bond energies
Question 3. The axial overlap between the two orbitals leads to the formation of a:
(a) sigma bond (b) pi bond (c) multiple bond (d) none of these
Question 4. In S02 molecule, S atom is:
(a)sp3 hybridized (b) sp hybridized (c) sp2 hybridized (d) d sp2 hybridized
Question 5. A molecule or ion is stable if:
(a) Nb = Na (b) Nb < Na (c) Na < Nb (d) Na – Nb = + ve
Question 6. The molecule Ne2 does not exist because
(a) Nb > Na (b) Nb = Na + (c) Nb < Na (d) None of these
Question 7. Which one is diamagnetic among NO+, NO and NO ?
(a)NO+(b) NO (c) NO–(d) None of these
Question 8. In sp3, sp2 and sp hybridized carbon atom, the p character is maximum in:
(a) sp3 (b) sp2
(c) sp (d) all of the above have same p-character
Question 9. Out of the following, intramolecular hydrogen bonding exists in:
(a) water (b)H2S (c) 4-nitrophenol (d) 2-nitrophenol -
-
المحادثة
-
منتدى
-
منتدى
-
الملف
-
الملف
-
-
-
المحادثة
-
الإفادة
-
منتدى
-
الملف
-
الملف
-
-
-
المحادثة
-
منتدى
-
الإفادة
-
الملف
-
-

1- CHERKAOUI EL MOURSLI Fouzia, RHALIB KNIAZEVA Albina, NABIH Khadija, l’Université Mohammed V de Rabat, Royaume du Maroc, 2015.
2- ELIZABET Bardez, chimie générale, Exercices et Problèmes, sciences sup, DUNOD, 2009.
3- CRISTOS COMNINELLIS, KLAUDE K.W. FRIEDLI, ARAKSI SAHIL-MIGIRDICYAN, Exercices de chimie générale, troisième édition revue et augmentée, 2010.
4- Richard Mauduit, Eric Wenner, Chimie générale en 30 fiches, Dunod, 2008.
5- Chimie générale, Raymond Chang, Kenneth A. Goldsby, Traduction et adaptation française Azélie Arpin, Luc Papillon, 4 eme Edition, 2014 McGraw-Hill Education.
6- Cours de Chimie Structure de la Matière, Dr Drola Zohra, Université Oran 2017-2018.
7- Structure de la Matière, Chimie 1 Cours et Exercices, Dr. Bendaoud Nadia, Université Oran 2015-2016.
8- Cours et Exercices de Structure de la Matière, Cours et exercices, Bey Said, Université A. MIRA – BEJAIA, 2015-2016.