TP Chemistry II (2023-2024)
Topic outline
-
-
Forum
-
-
Teacher: Dr. MAKHLOUFI El-Hani
Contact : elhani.makhloufi@univ-msila.dz
Availability at Laboratories: Tuesday-Thursday from 8:00 am - 5:00 pm
Course information :
Faculty : Technology
Department : COMMON BASE ST
target public : 1st year Licence
Course Title : TP Chimie 02
Credit : 02
Coefficient :01
Duration : 10 weeks
Hours : 02h00
Block: B
Room : Lab: 5,7,8 and 9.
2023-2024
-
These practical work sessions are designed for 1st year Common Base students. The Chemistry II practical work module consists of four manipulations:
- Manipulation N°1 : Calorific Capacity
- Manipulation N°2 : The Latent Heat of Fusion of the ice
- Manipulation N°3 : Determining enthalpies of reaction
- Manipulation N°4 : Enthalpy of neutralization (HESS law)
- Manipulation N°5 : The Heat capacity (Specific Heat)
- Manipulation N°6 : Chemical kinetics: AUTO-CATALYSIS
Each practical work session is accompanied by theoretical information, and questions are asked to check the acquisition and assimilation of knowledge. A report is submitted to the teacher within a maximum of 15 days from the date of the work session.
TP 01
 TP 02
TP 02 TP 03
TP 03 TP 04
TP 04 TP 05
TP 05
-
Thermodynamics is the science of energy exchange. It is used as much in physics as in chemistry and biology, relying in particular on mathematical tools. Its fundamental principles enable us to understand and predict energy variations between different interacting systems. Thermodynamics has many applications. These include thermal machines (car engines, refrigerators, heat pumps).
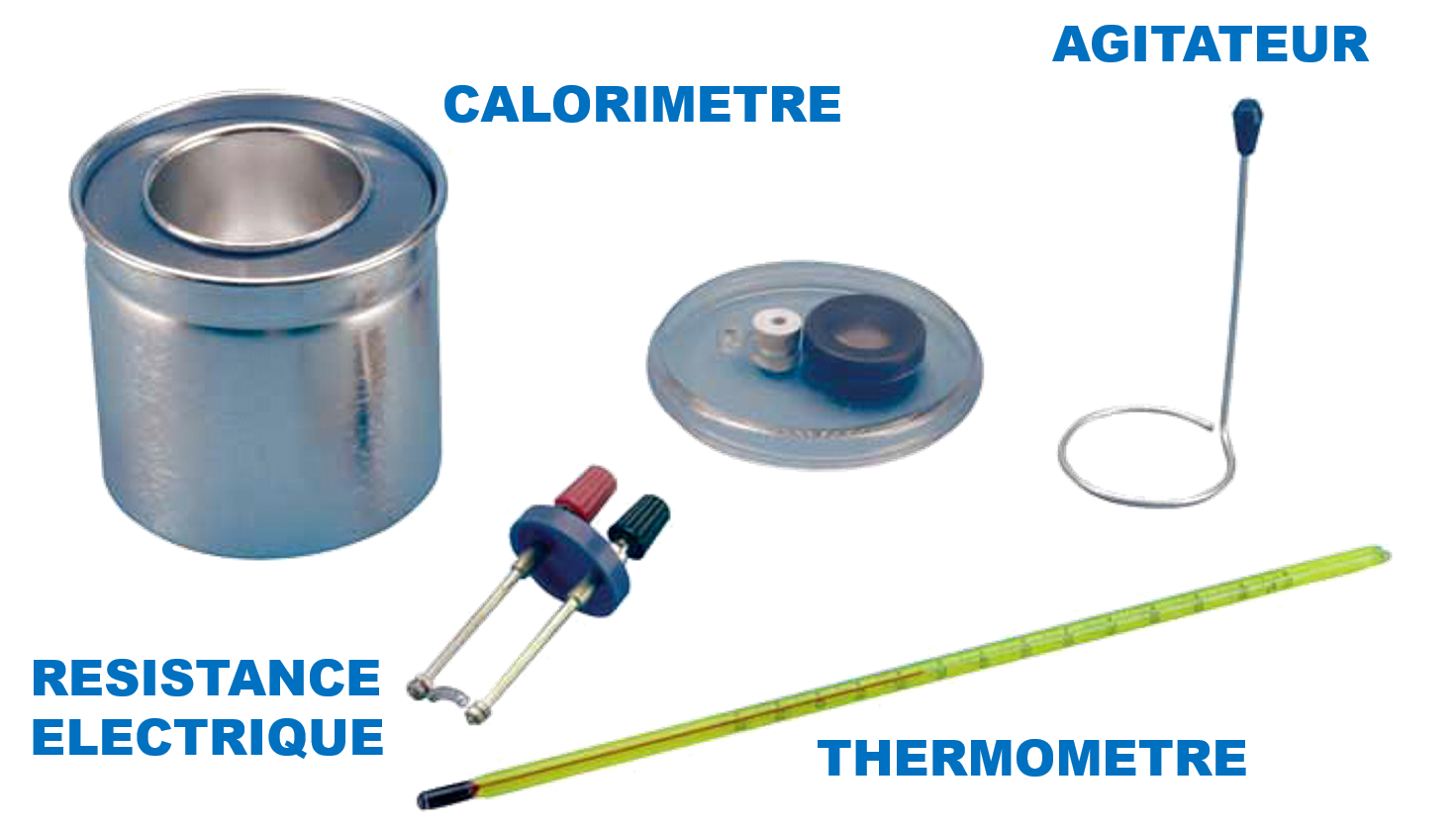
The calorific capacity C of a substance is the physical quantity that determines the amount of heat Q required to vary its temperature by a certain value ∆T. We know from experience that calorific capacity is an extensive property, in the sense that the calorific capacity of a given sample is directly proportional to its dimensions (it is faster to chauffer a glass of water than a barrel of water using the same heat source). The mass heat capacity is the calorific capacity related to the mass of the substance. It is an intensive quantity, because it depends only on the nature of the substance and not on its dimensions.
Objectives

1- Determine the calorific capacity of the calorimeter.
2- Determining the specific heat of a liquid (ethanol).
3- Determining the specific heat of a metal.
ENG
 FR
FR
-
Chat
-
Feedback
-
Forum
-
A given pure substance can exist in 4 states: solid, liquid, gas and plasma. The change of physical state requires an exchange of heat (energy) with the external environment.
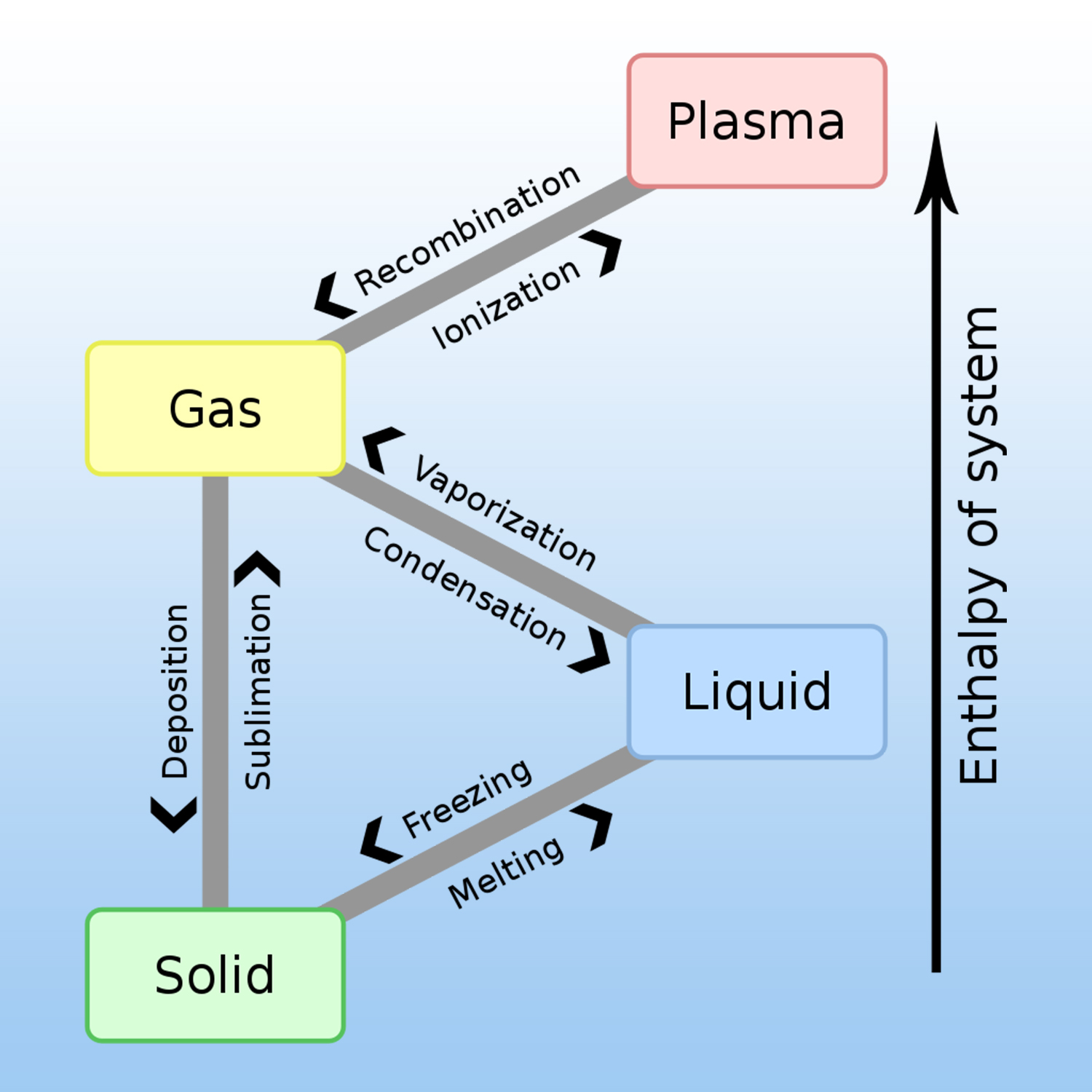
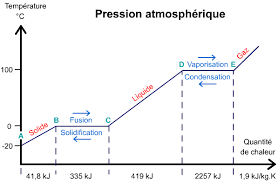
A physical change is produced when there is no transformation of matter, for example, liquid water that evaporates always remains water, H2O.
At constant pressure, once a pure substance (such as water) has reached its change-of-state temperature, it needs an additional quantity of energy to change state: this is the mass energy of change of state, also called the « latent heat of change of state », noted L. It is measured by the variation in thermal energy Q, such as :
Q = m . L m: the mass in Kg.
Objective

1- Study of change of state phenomenon.
2- Determination of the calorific capacity of the calorimeter (Ccal).
3- Determination of the latent heat of fusion of the ice (Lf).
ENG
 FR
FR
-
Chat
-
Feedback
-
Forum
-
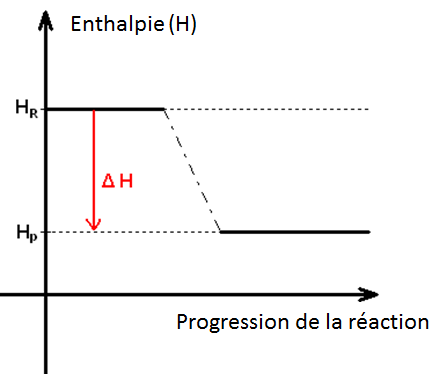
In any chemical reaction, heat is either absorbed or liberated. This exchange of heat between a chemical reaction and its immediate environment is known as the enthalpy of reaction or "H". This enthalpy cannot be measured directly, so scientists use the "variation" in temperature between the start and end of the reaction, which can then be used to calculate the "variation" in enthalpy over the same lapse of time (variation noted as ∆H). Using the ∆H, scientists are able to tell whether a reaction has been "exothermic" (losing or liberating heat during the reaction) or "endothermic" (absorbing heat). In general, a Constant Pressure ( ∆H = Q = m.c.∆T ) , with m representing the mass of the components, c the specific heat of the product and ∆T being the temperature variation during the reaction.
.
Objectives

1- Determine the calorific capacity of the calorimeter.
2- Measuring the enthalpy of dissolving KCl salt in water.
3- Measuring the enthalpy of diluting an HCl solution.
ENG
 FR
FR
-
Chat
-
Feedback
-
Forum
-
Chemical reactions are generally accompanied by the liberation or absorption of heat. If heat is liberated, the reaction is said to be exothermic. If heat is absorbed, the reaction is said to be endothermic.
Many chemical reactions take place at constant pressure, usually atmospheric pressure. In this case, we define a state function, called enthalpy H, to study the heat of reactions at constant pressure.
For a reaction taking place at constant pressure and for which no work is exchanged (other than that of the pressure forces), the change in enthalpy ΔH is equal to the quantity of heat exchanged Qp (ΔH = Qp).
By convention, ΔH and Qp are negative if the reaction is exothermic (energy is lost by the system).
ΔH and Qp are positive if the reaction is endothermic (energy is gained by the system).
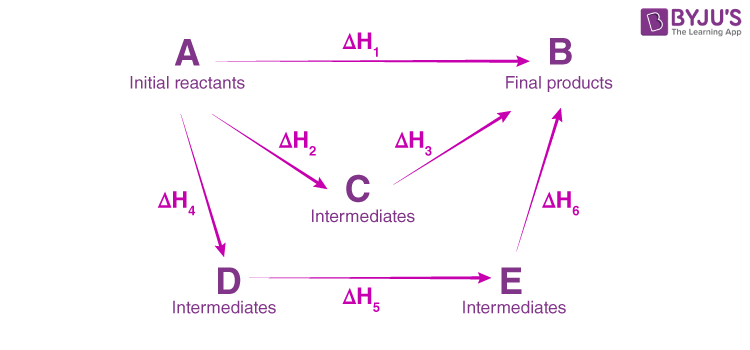
- Verification of the HESS law:
The enthalpy variations of a system depend only on the initial and final states, if the transformation is effected at constant volume or constant pressure. It does not depend on the path taken from the initial to the final state.
Consider the following transformations:
(1) NaOH(s) + (H+ + Cl-) ------------> (Na+ + Cl-) + H2O (ΔrH1)
(2) NaOH(s) ------------> (Na+ + OH -) (ΔrH2)
(3) (Na++ OH-) + (H++ Cl-) ------------> ( Na+ + Cl-) + H2O (ΔrH3)
Note that reaction (1) is the sum of reactions (2) and (3). By measuring the heats of the different transformations separately using calorimetry, we can verify that :
ΔrH1 = ΔrH2 + ΔrH3
Objectives

1- Determine the calorific capacity of the calorimeter.
2- Measure the heat of neutralisation of a strong acid by a strong base.
3- Check Hess's law.
ENG
 FR
FR
-
Chat
-
Feedback
-
Forum
-
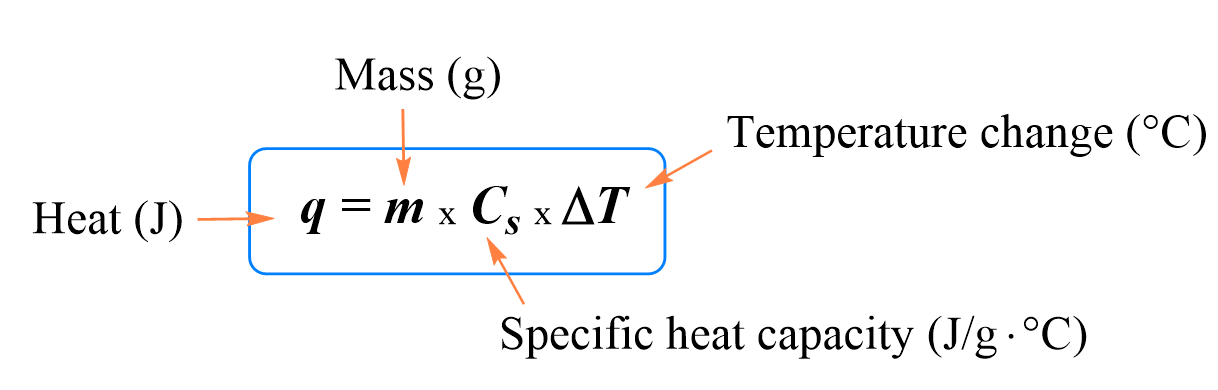
The specific heat capacity at constant pressure is the quantity of energy that must be supplied to one kilogram of the body in consideration to raise its temperature by one degree K (or °C), while keeping its pressure constant.
The unit in the international system is the joule per kilogram kelvin, J·kg-1·K-1 The determination of the heat capacities of substances is known as calorimetry.
· The calorific capacity of a calorimeter is generally very low, of the order of a few tens of Joules. It is an experimental quantity which can be determined from measurements and calculations of the type described in the paragraph " Examples of calorimetric calculations, Calculation of the final temperature of a system when 2 miscible liquids are mixed ".
· The quantity of heat exchanged dQ for a variation in temperature dT of a body is proportional to the mass m of the body and its mass heat c.
(δQ = m.c.dT)
ENG
 FR
FR
-
Chat
-
Feedback
-
Forum
-
La cinétique chimique a pour objet d’étudier la vitesse de formation, de proposer des mécanismes, donc de définir des chemins réactionnels.
Les réactions chimiques ne sont pas toutes égales face au temps : certaines réactions sont si rapides comme les réactions acido-basiques, d'autres sont très lentes comme par exemple la formation du pétrole .
Bien avant de s'intéresser aux constituants de la matière — atomes ou molécules — les chimistes se sont posés la question de ces différences : comment expliquer qu'une réaction (par exemple, une estérification) prend 23 ans à se réaliser dans la nature, mais se complète en un quart d'heure (15 min) si on chauffe et on acidifie le milieu ?
Avant même de pouvoir répondre, il faut se poser une question encore plus élémentaire : comment mesurer la « vitesse » d'une transformation chimique ?
Objectifs

- Étude cinétique de l’évolution d’un système par analyse chimique « en continu ».
- Quel est le rôle d'un catalyseur (Autocatalyse).

-
Chat
-
Feedback
-
Forum
-

1. Lucien Borel, Daniel Favrat, Thermodynamique et énergétique, PPUR, 2005.
2. Jacques Mesplède, Jérôme Randon, 100 manipulations de chimie générale et analytique, Editions Bréal, 2004.
3. John C. Kotz, Paul M. Treichel Jr, Chimie des solutions, De Boeck Supérieur, 2006.
